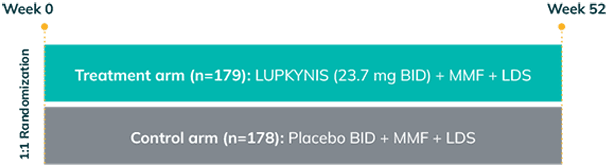
AURORA 1 was a randomized, double-blind, placebo-controlled, phase 3 study in adults with class III or IV (±V) or class V LN that assessed the proportion of patients achieving complete renal response (CRR) at Week 52 (primary endpoint) with LUPKYNIS compared with MMF + low-dose steroids alone1,2,a
AURORA 1: 52 weeks (N=357)
Key inclusion criteria1:
- Active LN class III or IV (±V) or class V
- UPCR ≥1.5 mg/mg (≥2 mg/mg class V)
Key exclusion criteria1:
- eGFR ≤45 mL/min/1.73 m2
MMF + steroid dosing1:
- Oral MMF at a target dose of 2 g/day
- Intravenous methylprednisolone 0.25-0.5 g/day on Days 1 and 2b
- Oral steroid initiated on Day 3 with 20-25 mg/day prednisone and rapidly tapered to a target dose of 2.5 mg/day at Week 16b
Primary efficacy endpoint1:
- Proportion of patients achieving complete renal response at Week 52
LUPKYNIS was studied with stringent criteria that robustly defines complete renal response as1,c:
- UPCR ≤0.5 mg/mg
- eGFR ≥60 mL/min/1.73 m2 or no confirmed decrease from baseline in eGFR >20% or no treatment- or disease-related eGFR-associated eventd
- Sustained low-dose steroidsc
- No administration of rescue medications
aMMF + low-dose steroids alone refers to placebo + MMF + low-dose steroids.
bOn Days 1 and 2 (0.5 g/day for patients ≥45 kg and 0.25 g/day for patients <45 kg). All patients began taper of oral prednisone on Day 3, decreasing over time to 2.5 mg/day at Week 16 based on eGFR and blood pressure in a predefined dosage adjustment protocol.1
cIn order to be considered a responder, the patient must not have received more than 10 mg/day prednisone for ≥3 consecutive days or for ≥7 days in total during Weeks 44 through 52. Patients who received rescue medication or withdrew from the study were considered nonresponders.1
dDefined as blood creatinine increased, creatinine renal clearance decreased, glomerular filtration rate decreased, serum creatinine increased, renal impairment, renal failure, or renal failure acute.1
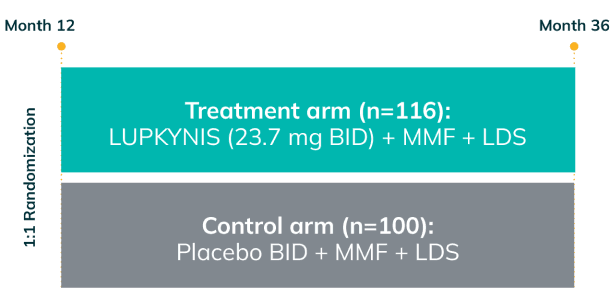
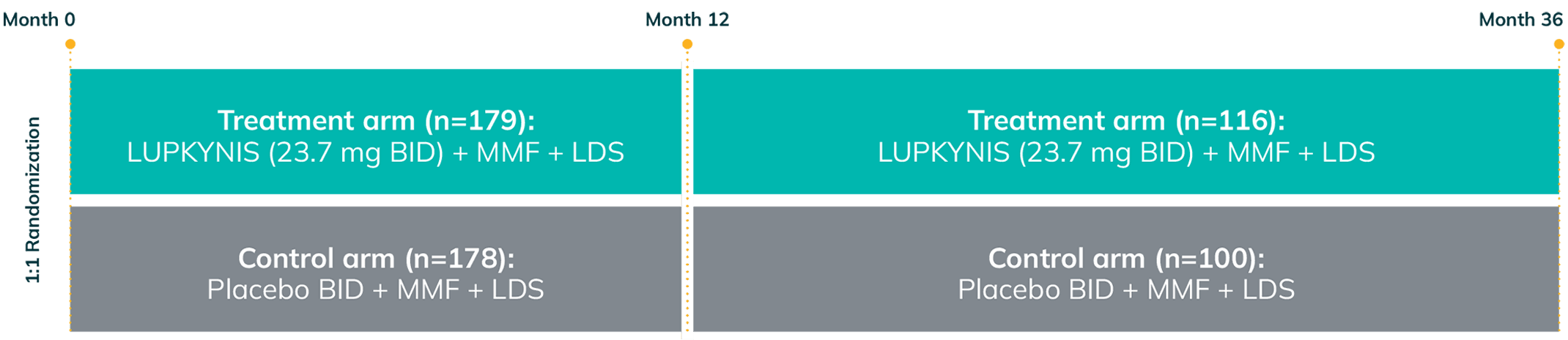
AURORA 2: Two Additional Years of Study Treatment—One of the Longest and Largest Blinded Data Sets in LN1,3
AURORA 2 assessed the long-term safety and tolerability (primary endpoint) and long-term efficacy (secondary endpoint) of LUPKYNIS compared with MMF + low-dose steroids alonea for up to an additional 24 months following completion of 12 months of treatment in AURORA 11,3
- Patients who completed AURORA 1, and who elected to continue, remained in the same blinded treatment group1,3
- Over 52% of the 357 patients completed AURORA 1 (LUPKYNIS 56.4%, active control 47.8%); 51.4% of LUPKYNIS patients and 41.0% of active control patients were still on study treatment at the end of AURORA 21,3
AURORA 1: 52 weeks (N=357)
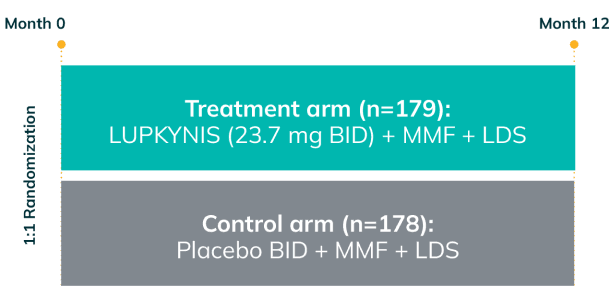
AURORA 2: 2-year, blinded continuation (N=216)
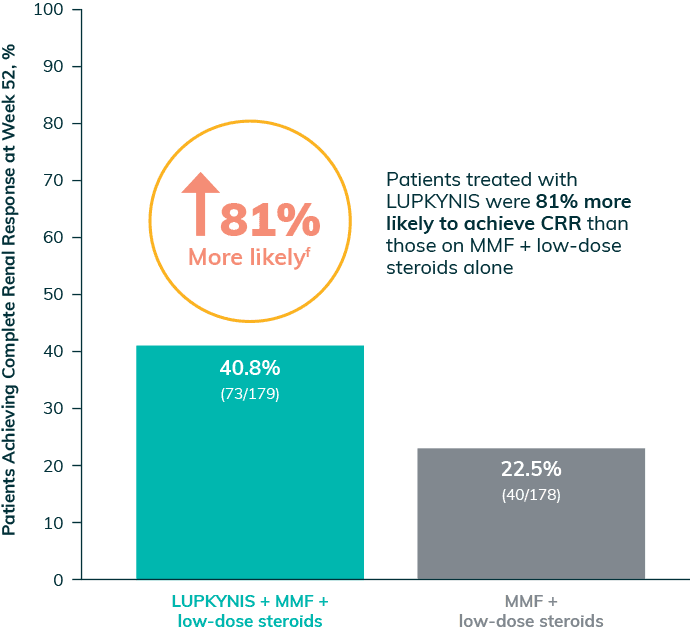
Significantly More Patients on LUPKYNIS Achieved a Complete Renal Response (CRR)1
Complete Renal Response at Week 52e
(primary endpoint; P<0.001)
51%
of newly diagnosed patients receiving LUPKYNIS achieved a complete renal response at 1 year (compared with 28% of patients receiving MMF + low-dose steroids alone)4,g
IMPORTANT NOTE: This is a post hoc analysis and should be interpreted with caution.4
The ACR recommends triple immunosuppressive therapy, including CNI treatment such as LUPKYNIS, as first-line therapy, with the goal of treatment being the preservation of kidney function.5
aMMF + low-dose steroids alone refers to placebo + MMF + low-dose steroids.
eStringent criteria of complete renal response as: UPCR of ≤0.5 mg/mg, maintained stable eGFR, sustained low-dose steroids, and no administration of rescue medications. Click here for additional information on primary endpoint.1
f81% more likely calculation: ([40.8 – 22.5]/22.5) x 100 = 81%.1
gPost hoc analysis of patients with recent-onset LN—excluding class V—defined as LN diagnosis within ≤6 months based on reported year of diagnosis, study start date, and date of biopsy4
Complete Renal Responses At 1 Year for LUPKYNIS vs Active Control6,h
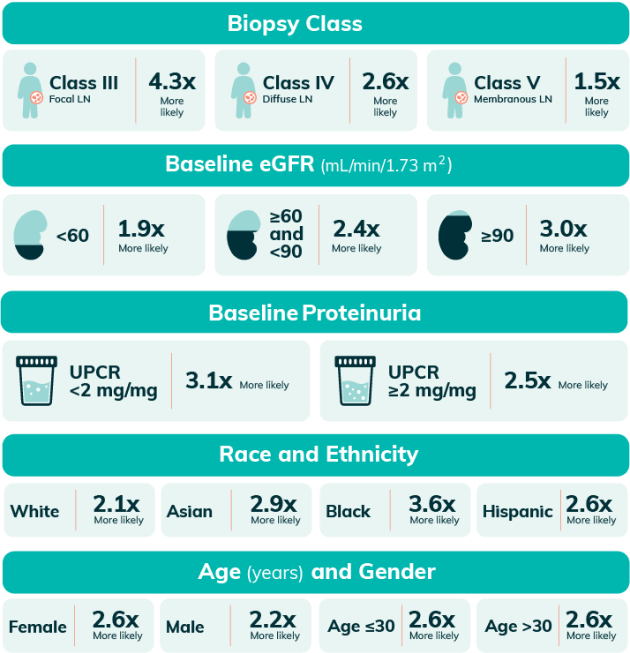
Complete Renal Responses Across Biopsy Class and Baseline Conditions Between LUPKYNIS vs Active Control6,h
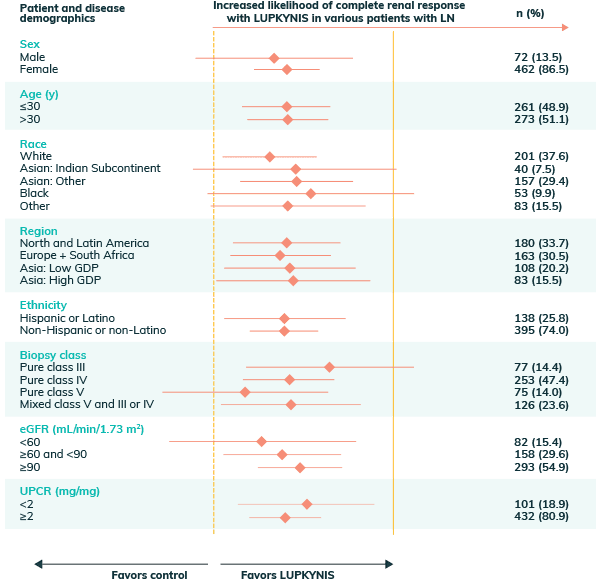
hPooled analysis of complete renal response at 1 year includes integrated data from AURA-LV and AURORA 1. Analysis uses a logistic regression model with covariates for study, treatment group, subgroup, and treatment by subgroup interaction6
LUPKYNIS Rapidly Reduced Proteinuria in Fewer Days2
LUPKYNIS Reduced Proteinuria 2x Faster Than MMF + Low-Dose Steroids Alone2,a
UPCR ≤0.5 mg/mg in <6 Months2,i
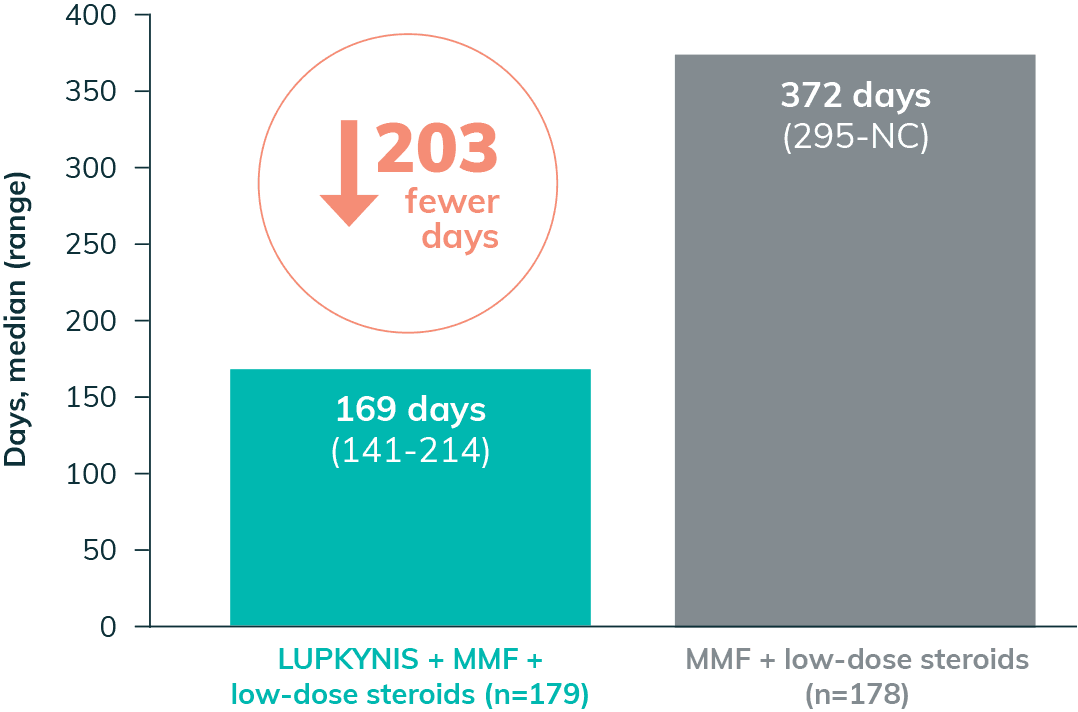
- The median time to UPCR ≤0.5 mg/mg with LUPKYNIS was 169 days vs 372 days with MMF + low-dose steroids (HR: 2.0; 95% CI: 1.5-2.7)2
50% UPCR Reduction in 29 Days2,i
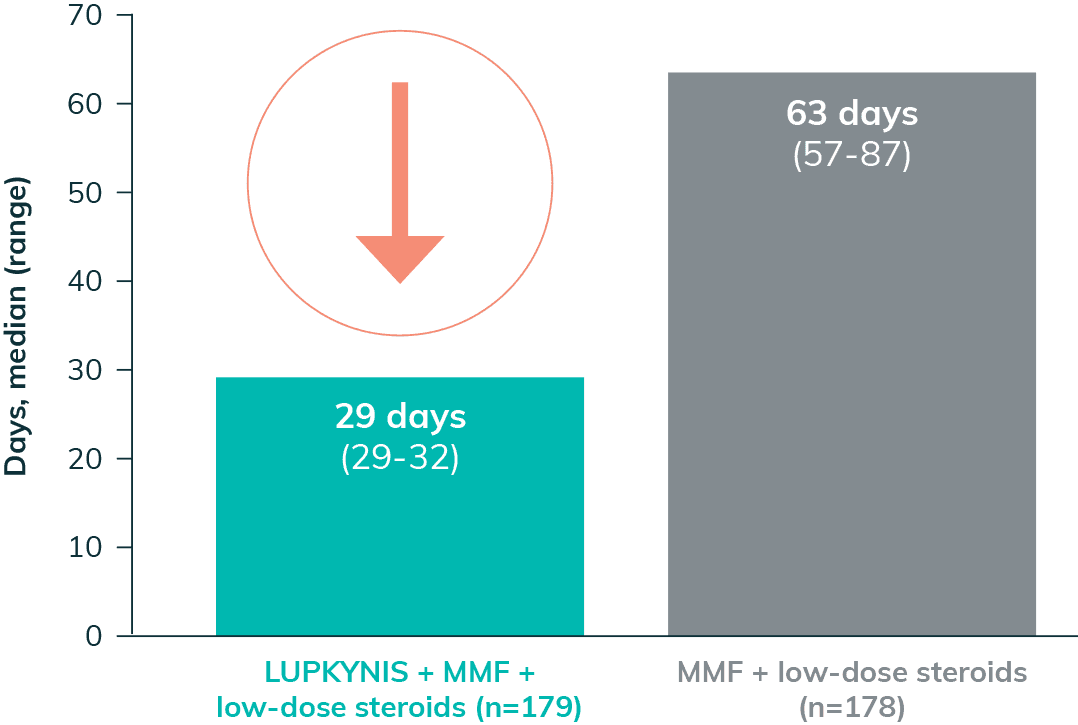
- The median time to 50% UPCR reduction with LUPKYNIS was 29 days vs 63 days with MMF + low-dose steroids2
The ACR-recommended goal of therapy is to reach proteinuria ≤0.5 g/g by 6-12 months.5
aMMF + low-dose steroids alone refers to placebo + MMF + low-dose steroids.
iSecondary endpoint.2
Stabilized eGFR and Preserved Kidney Function Throughout the 3-Year Clinical Program2,3
Stable Mean Corrected eGFR Across All the Time Points Throughout the 3-Year Clinical Program With LUPKYNIS2,3
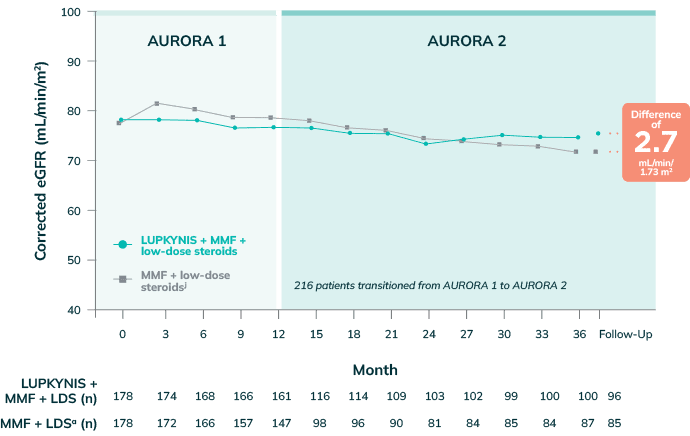
Adapted with permission from Saxena A et al. Arthritis Rheumatology. 2024;76(1):59-67.
The ACR‑recommended goal of therapy is to reach stabilization or improvement in kidney function ±20% baseline within 6-12 months.5
- During AURORA 2, the LS mean corrected eGFR values increased by 1.4 mL/min/1.73 m2 in the LUPKYNIS arm while corrected eGFR value dropped by 3.3 mL/min/1.73 m2 in the active control arm7
- Across the 3 years of study, a good renal outcome, based on adjudicated adequate renal response (defined as UPCR ≤0.7) and no renal flare, was achieved by 66.4% of patients treated with LUPKYNIS + MMF + low-dose steroids compared with 54.0% of patients who received active control3
- Approximately 2x more patients treated with LUPKYNIS + MMF + low-dose steroids (20.1%) achieved a sustained complete renal response through Year 3 compared with those treated with active control (11.8%)1,k
- Reductions in proteinuria with LUPKYNIS + MMF + low-dose steroids persisted through 3 years3,l
eGFR should be monitored periodically; please see Dosing for more information.
aMMF + low-dose steroids alone refers to placebo + MMF + low-dose steroids.
jKidney function assessed with corrected eGFR (Chronic Kidney Disease Epidemiology Collaboration equation) using a prespecified ceiling of 90 mL/min/1.73 m2. Analysis of AURORA 2 patients includes data from the AURORA 1 pretreatment baseline, 12 months in AURORA 1, and up to 24 months in AURORA 2.3
kDefined as achieving renal response at Month 12 of AURORA 1 and maintaining renal response at subsequent clinic visits through the end of the extension study at Month 36.1
lReductions in proteinuria were defined as reductions in mean UPCR achieved during the first year of treatment in AURORA 1.3
Annualized Slope of Corrected eGFR Was Stable With LUPKYNIS Compared to Active Control3,m
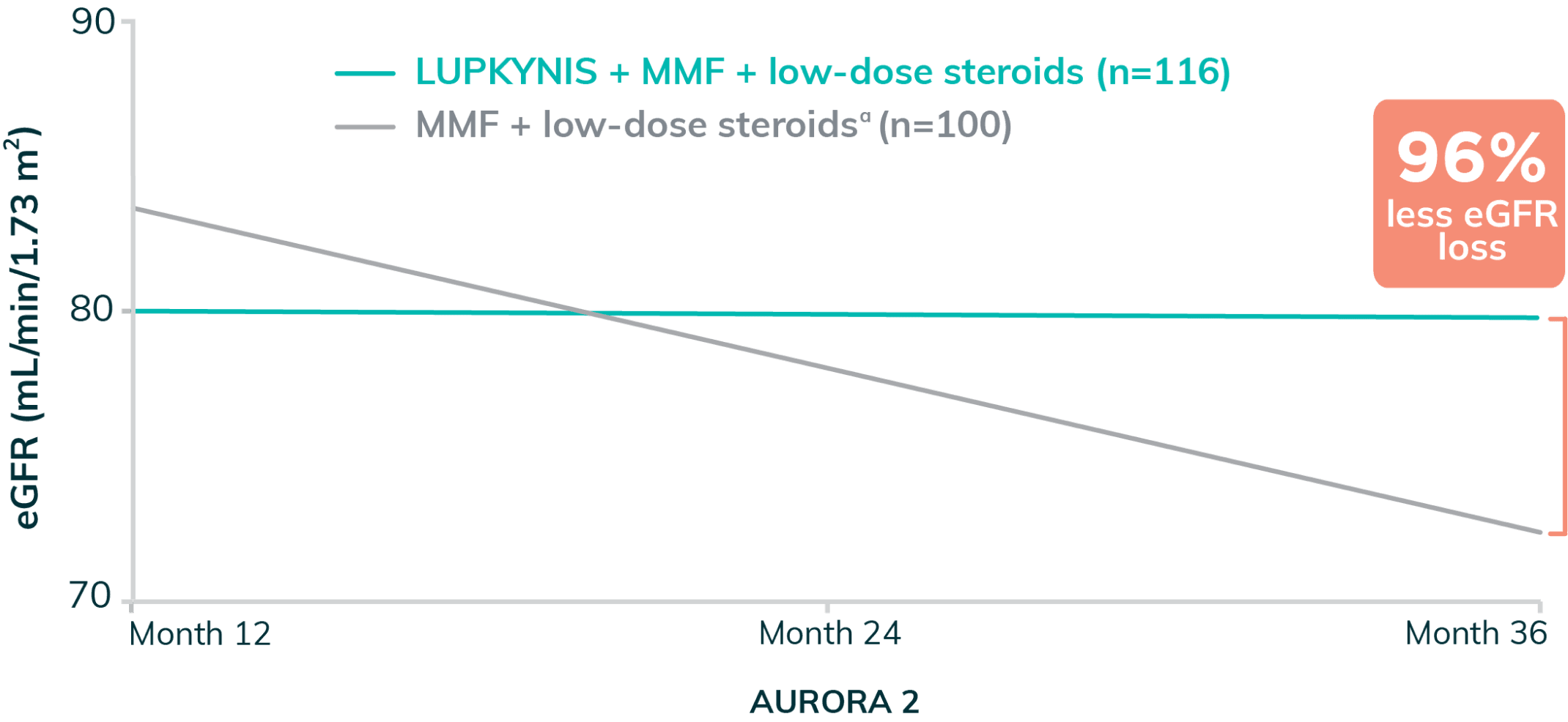
Adapted with permission from Saxena A et al. Arthritis Rheumatology. 2024;76(1):59-67.
- Long-term renal function was evaluated by calculating the eGFR slope throughout 24 months in AURORA 2. From Month 12 to Month 36, the annualized corrected eGFR slope was -0.2 mL/min/1.73 m2 (95% CI: -3.0 to 2.7) with LUPKYNIS + MMF + low-dose steroids and -5.4 mL/min/1.73 m2 (95% CI: -8.4 to -2.3) with MMF + low-dose steroids3
- LUPKYNIS treatment was associated with a stable eGFR from Month 12 to Month 36 (-0.2 mL/min/1.73 m2) compared with a substantial decline with MMF + low-dose steroid alone (-5.4 mL/min/1.73 m2). Mean serum creatinine levels remained within normal range over 3 years with LUPKYNIS3,n
Annualized change in eGFR with LUPKYNIS + MMF + low-dose steroids represents 96% less eGFR loss throughout AURORA 2, compared with placebo + MMF + low-dose steroids alone3,a
aMMF + low-dose steroids alone refers to placebo + MMF + low-dose steroids.
mThese changes in slope are annualized based on generalized linear model analysis of individual patient slopes.3
nPercent difference in eGFR loss calculation: 100 – ([-0.2/-5.4] x 100) = 96.3%3
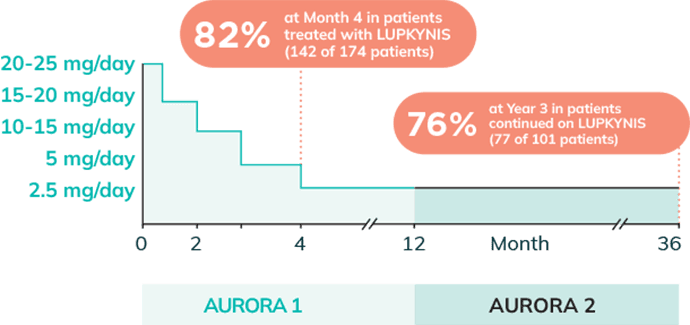
LUPKYNIS Efficacy Was Achieved With a Steroid-Sparing Study Protocol Tapered to ≤2.5 mg/day3
Approximately 80% of Patients Treated With LUPKYNIS Were Tapered to and Maintained on Low Doses of Steroids (≤2.5 mg/day) Through Year 33
Rapid and sustained steroid reductions to ≤2.5 mg/day (per AURORA 1 and AURORA 2 protocols)2,3
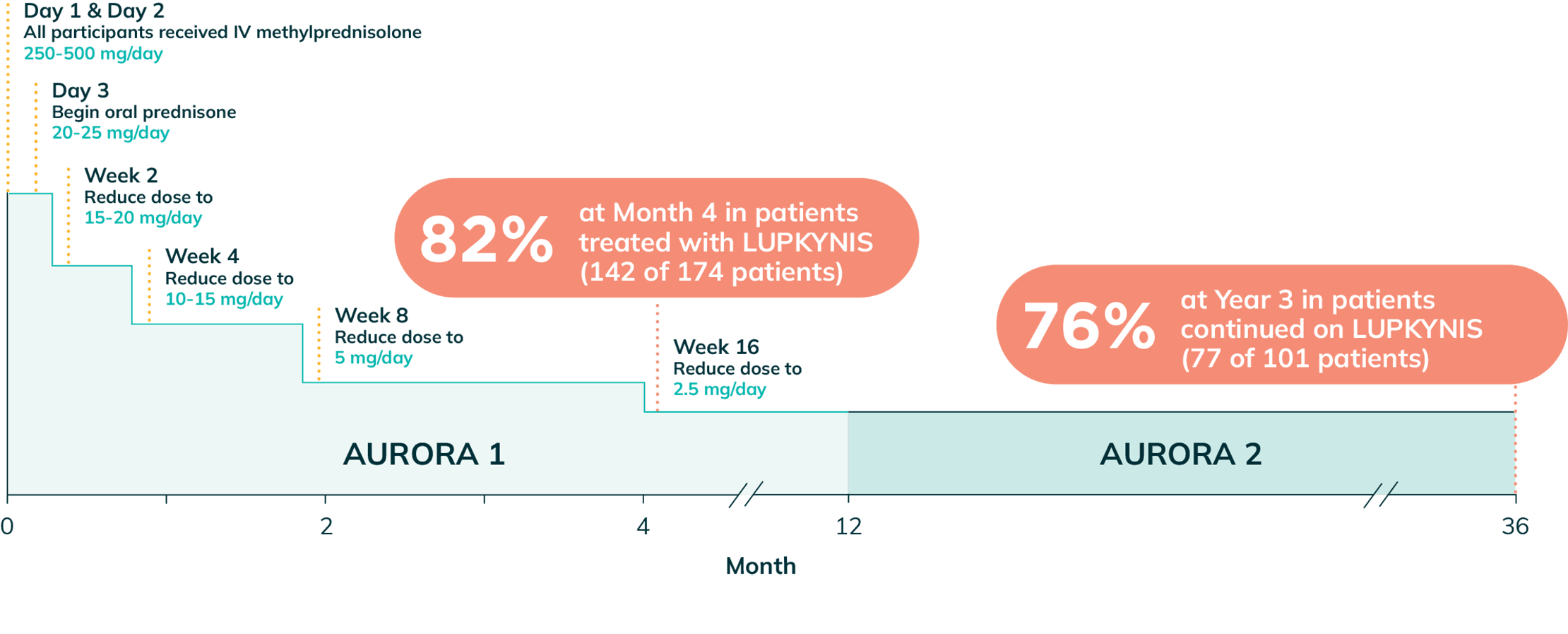
Day 1 & Day 2
All participants received IV methylprednisolone
250-500 mg/day
Day 3
Begin oral prednisone
20-25 mg/day
Week 2
Reduce dose to
15-20 mg/day
Week 4
Reduce dose to
10-15 mg/day
Week 8
Reduce dose to
5 mg/day
Week 16
Reduce dose to
2.5 mg/day
- Study included scheduled steroid taper from 20 to 25 mg/day at Week 1 to 2.5 mg/day by Week 161,2,o,p,q
- Per protocol, steroid doses were tapered lower than guideline recommendations in both study groups2,8,r,s
The ACR recommends tapering steroid to ≤5 mg/day within 6 months.5
oThe mean (SD) daily dose of intravenous methylprednisolone on Day 1 was 495 (±90) mg/day and Day 2 was 487 (±55) mg/day. At Week 16, 142 (82%) of 174 patients in the LUPKYNIS arm and 138 (81%) of 171 patients in the active control arm were on an oral steroid dose of ≤2.5 mg/day. At Week 52, 121 (75%) of 162 patients in the LUPKYNIS arm and 108 (73%) of 147 patients in the active control arm were on an oral steroid dose of ≤2.5 mg/day. At Month 36, 77 (76.2%) of 101 patients in the LUPKYNIS arm and 66 (77.6%) of 85 patients in the active control arm were on an oral steroids dose of ≤2.5 mg/day.2
pPatients with a lack of response were allowed one 4-week interval without dose reduction or one dose escalation to the previous dose for 2 weeks at any time during the study. Lack of response was defined as no or minimal change in UPCR per investigator judgment over 3 visits or deterioration in UPCR not meeting the criteria for withdrawal.7
qPatients who completed AURORA 1, and who elected to continue, remained in the same blinded treatment group. Patients in AURORA 2 had up to an additional 24 months of total exposure.3
r0.5 g/day (body weight ≥45 kg) or 0.25 g/day (body weight <45 kg).2
s20 mg/day for subjects <45 kg and 25 mg/day for subjects ≥45 kg.1,2
BID=twice daily; CI=confidence interval; CRR=complete renal response; eGFR=estimated glomerular filtration rate; GDP=gross domestic product; HR=hazard ratio; LDS=low-dose steroids; LN=lupus nephritis; LS=least squares; MMF=mycophenolate mofetil; NC=non-calculable; OR=odds ratio; UPCR=urine protein-to-creatinine ratio.
References: 1. LUPKYNIS®. Prescribing information. Aurinia Pharma U.S., Inc.; 2025. 2. Rovin BH, Teng YKO, Ginzler EM, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021;397(10289):2070-2080. 3. Saxena A, Ginzler EM, Gibson K, et al. Safety and efficacy of long-term voclosporin treatment for lupus nephritis in the phase 3 AURORA 2 clinical trial. Arthritis Rheumatol. 2024;76(1):59-67. 4. Mackay M, Truman M, England N, Birardi V, Mina-Osorio P. Efficacy of voclosporin in recent onset lupus nephritis. Arthritis Rheumatol. 2021;73(Suppl 10). Abstract 1752. 5. 2024 American College of Rheumatology (ACR) Guideline for the Screening, Treatment, and Management of Lupus Nephritis: Guideline Summary. American College of Rheumatology. November 18, 2024. Accessed November 18, 2024. 6. Arriens C, Teng YKO, Ginzler EM, et al. Update on the efficacy and safety profile of voclosporin: an integrated analysis of clinical trials in lupus nephritis. Arthritis Care Res. 2023;75(7):1399-1408. 7. Aurinia Pharma U.S., Inc. Data on file. 8. Fanouriakis A, Kostopoulou M, Andersen J, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. 2024;83(1):15-29.