LN Is Among the Most Severe and Dangerous Complications of Systemic Lupus Erythematosus (SLE)1,2
SLE Affects Approximately 200,000 to 300,000 Patients in the United States7-11
ONLY
42%
of patients with SLE are screened for LN12,a
UP TO
60%
of patients with SLE develop LN13
87%
of patients with LN are class III, IV, or V2
ONLY
42%
of patients with SLE are screened for LN12,a
UP TO
60%
of patients with SLE develop LN13
87%
of patients with LN are class III, IV, or V2
By the time LN is diagnosed, irreversible kidney damage may already be present.14
UP TO
30%
of patients with LN progress to end-stage
renal disease within 15 years of diagnosis3
45x
increased risk of kidney failure once
diagnosed with LN2,b,c
32.8
YEARS
mean age of patients with LN who
submitted to kidney transplantation15
aPatients with SLE without LN.
bAs compared with patients with SLE without LN.2
cAnalysis of Systemic Lupus International Collaborating Clinics inception cohort of newly diagnosed patients enrolled between 1999 and 2012, who were followed for a mean of 4.6 years. A total of 1827 patients were recruited, of whom 700 had LN over the course of follow-up.2
Proteinuria Is a Significant Risk Factor for Kidney Damage16,17
Even low levels of proteinuria may be associated with significant kidney damage16,17
- Patients with proteinuria <0.5 g/day had a median chronicity index of 2 (range: 0-4)14
- Proteinuria level at 12 months is a predictor of long-term renal outcomes18,d
- Levels of proteinuria 0.5-0.8 g/day predict long-term renal outcomes, including 15-year CKD-free survival18-20

Did you know? Even mild clinical presentations (eg, subnephrotic proteinuria) can be associated with active histological lesions21,22
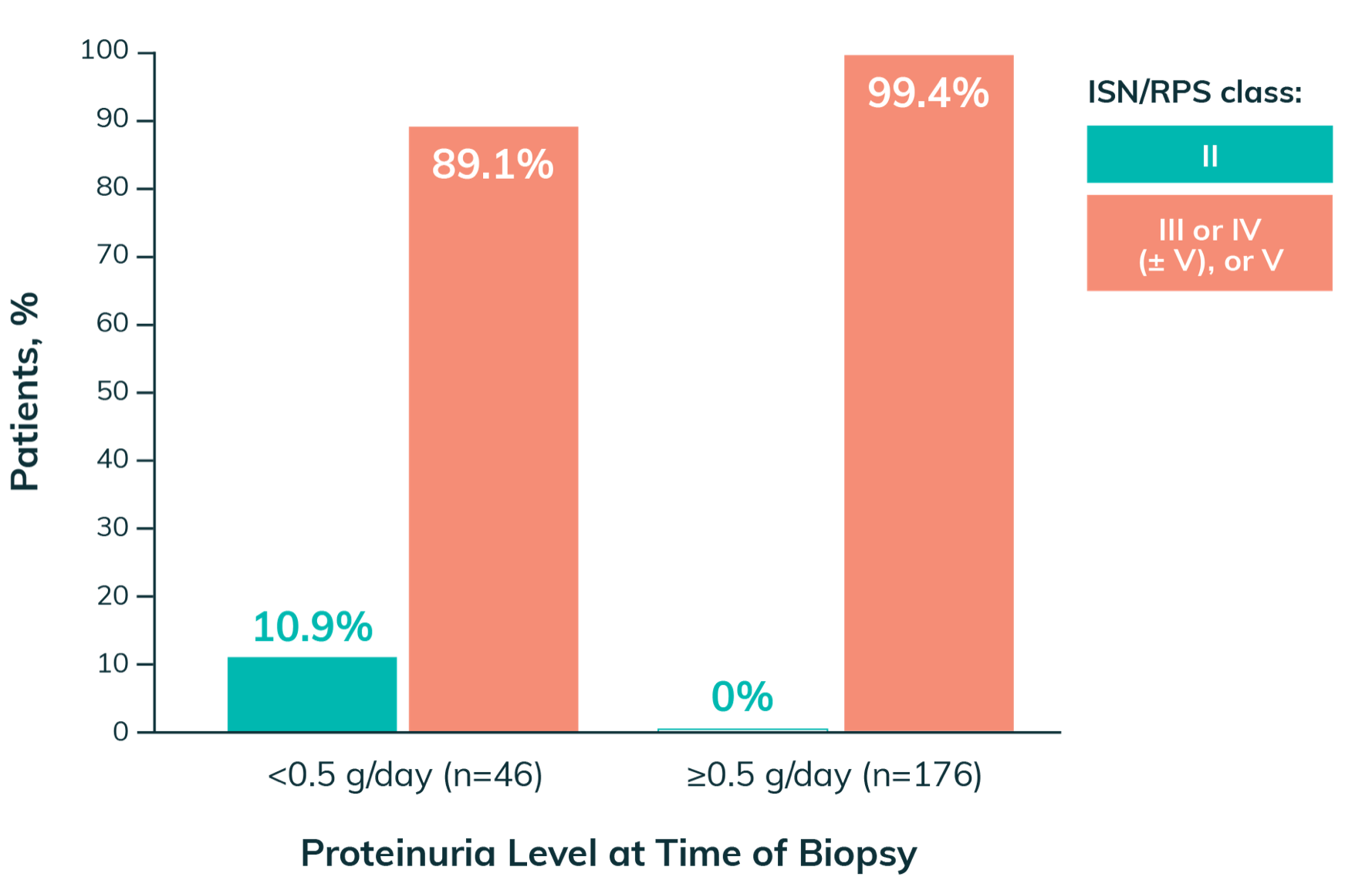
Nearly 90%
of patients with proteinuria ≤0.5 g/day have been reported to have class III or IV (±V) LN on biopsy14
Histologic Findings at Biopsy in Patients With SLE14,e-g
Even a Single Flare of LN Can Reduce the Lifespan of the Kidney6
Nephron Loss and LN
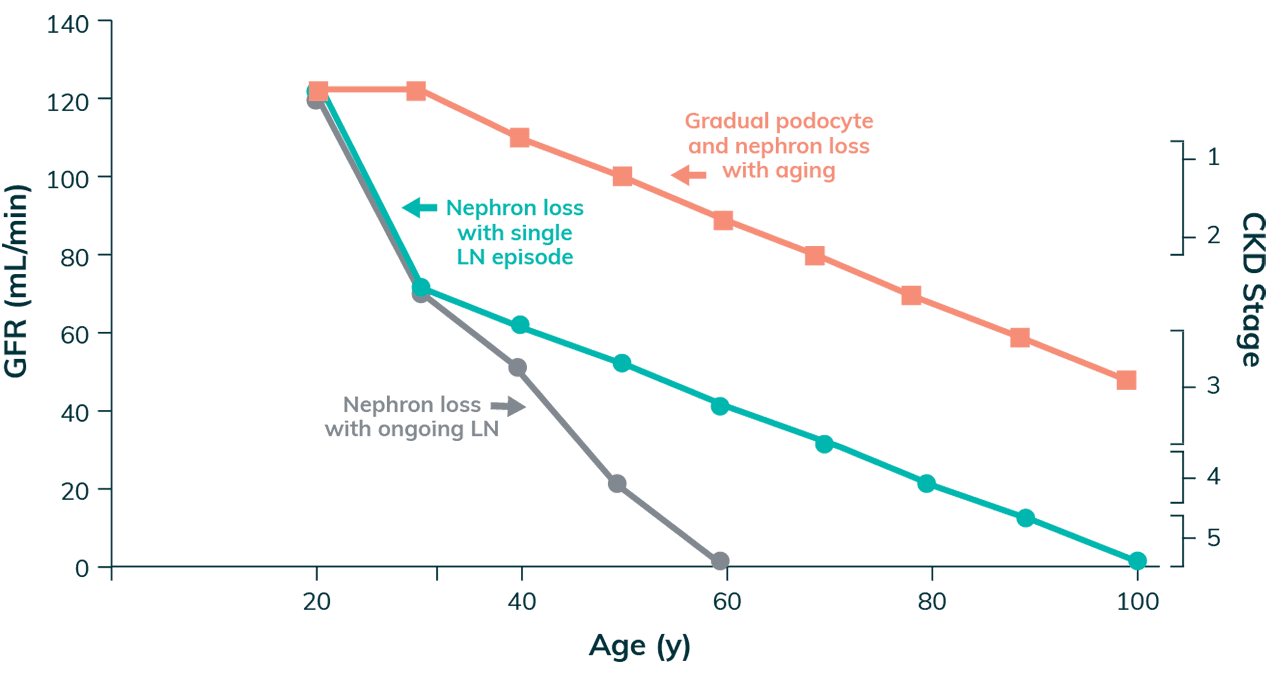
Adapted with permission from Anders HJ et al. Nature Reviews Disease Primers. 2020;6(1):7.
- A single flare of LN can cause irreversible nephron loss, potentially shortening the lifespan of the kidneys by decades6
- Every subsequent flare contributes to the accrual of kidney damage, further shortening kidney lifespan and increasing the risk of adverse long-term outcomes such as ESKD6
- Nephron loss and podocyte damage often lead to protein leakage into the urine6,23
- Proteinuria as a marker of kidney damage routinely precedes eGFR decline6,16

Early and definitive treatment is crucial to preserving nephron loss6
dRetrospective analysis conducted to determine if proteinuria was a good predictor of renal outcomes in a racially diverse group of patients with severe LN. A total of 107 patients with biopsy-proven LN with at least 7 years of available longitudinal follow-up data were selected for the study, of which 94 patients diagnosed with end-stage renal disease after the first year of follow-up were enrolled. Poor long-term renal outcome was defined as serum creatinine ≥1.5 mg/dL at the 7-year follow-up, and good renal outcome was serum creatinine <1.5 mg/dL. Kaplan-Meier curves were used to assess renal survival using the proposed proteinuria cutoff of <0.8 g/24 hours at 12 months.18
eFrom a study of 222 patients with SLE who underwent kidney biopsy for suspicion of LN.14
fPercentage of patients with ISN/RPS class III or IV (± V), or V with proteinuria <0.5 g/day calculation: ([14 + 21 + 2 + 4 + 0]/46) x 100 = 89.1%.14
gPercentage of patients with ISN/RPS class III or IV (± V), or V with proteinuria ≥0.5 g/day calculation: ([18 + 135 + 3 + 19]/176) x 100 = 99.4%.14
Proteinuria Reduction Is Associated With Long-Term Renal Protection24
Kidney Survival Based on Proteinuria Response Status24,h

Adapted with permission from Chen YE et al. Clinical Journal of the American Society of Nephrology. 2008;3(1):46-53.
The larger the initial reduction in proteinuria in the first several months of management, the lower the risk of ESKD24
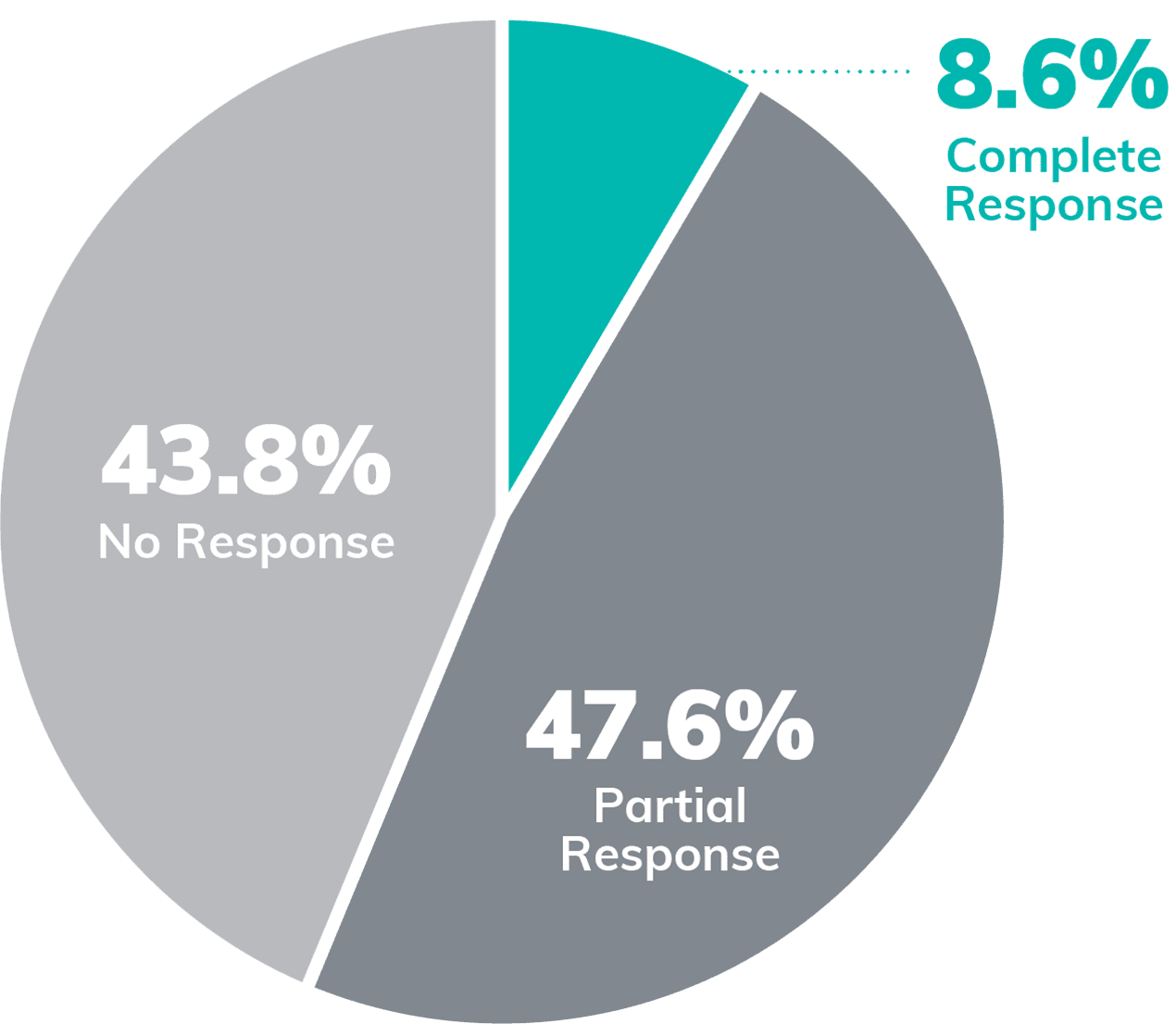
MMF + Steroids Induction Response at 24 Weeks25,i,j
The Need Remains for Additional Treatment Options5
MMF + steroidsk alone frequently fail to substantially reduce proteinuria, with only 20%-30% of patients achieving a complete response at 1-2 years5,26
hRetrospective analysis of patients (N=86) enrolled in the prospective controlled trial of plasmapheresis in severe LN to determine long-term prognosis of achieving partial response. Complete response was defined as SCr ≤1.4 mg/dL and proteinuria ≤0.33 g/day within 5 years of study entry, and partial response was defined as ≤25% increase in baseline SCr and ≥50% reduction in baseline proteinuria to ≤1.5 g/day (but >0.33 g/day) within 5 years of entering the study. Kidney survival was determined by kidney failure (≥6 mg/dL SCr or the initiation of kidney replacement therapy).24
iPercentage of patients with partial response calculations: 56.2% – 8.6% = 47.6%.25
jPercentage of patients with no response calculations: 100% – 56.2% = 43.8%.25
kIn the Aspreva Lupus Management Study, MMF (data presented here) and intravenous cyclophosphamide (data not shown) were compared, both with steroids, as induction treatment in patients with class III, IV, and V LN (N=370). Primary endpoint was response at 24 weeks. Response was defined as a decrease in UPCR to <3 mg/mg in patients with baseline nephrotic proteinuria (≥3 mg/mg), or by ≥50% in patients with subnephrotic baseline proteinuria (<3 mg/mg), and stabilization (±25%) or improvement in SCr as adjudicated by a blinded Clinical Endpoints Committee. Complete response was a secondary endpoint defined as return to normal SCr, urine protein ≤0.5 g/day, and inactive urinary sediment (≤5 WBC/hpf and ≤5 RBC/hpf, and a reading of lower than 2+ on dipstick and absence of red cell casts).25
2024 ACR Guideline for Screening, Treatment, and Management of LN
Select ACR Recommendations27
- Screening for proteinuria at least every 6-12 months and emphasis on the importance of prompt biopsy
- Treatment to reduce inflammation in the kidneys with a triple immunosuppressive regimen with first-line use of advanced therapies like LUPKYNIS for 3-5 years
- Tapering glucocorticoid to ≤5 mg/day by 6 months of therapy
- Shared decision-making with patients regarding treatment options and close monitoring to help reach treatment goals
Guideline Recommendations for Clinical Investigation and Management of LN
The 2024 ACR Guideline, 2024 KDIGO Guideline, and 2023 EULAR Guideline for LN Recommend5,13,27
Strive for early LN identification, given the repercussions of delaying diagnosis
Maintain close monitoring of your patients to ensure treatment goals are met
Once identified, consider early intervention with multitarget treatment regimen
LUPKYNIS Exceeds Guideline Goals With Rapid Reduction of Proteinuria and Steroid Doses1-6
The 2024 ACR Guideline, 2024 KDIGO Guideline, and 2023 EULAR Guideline for LN Recommend5,13,27:
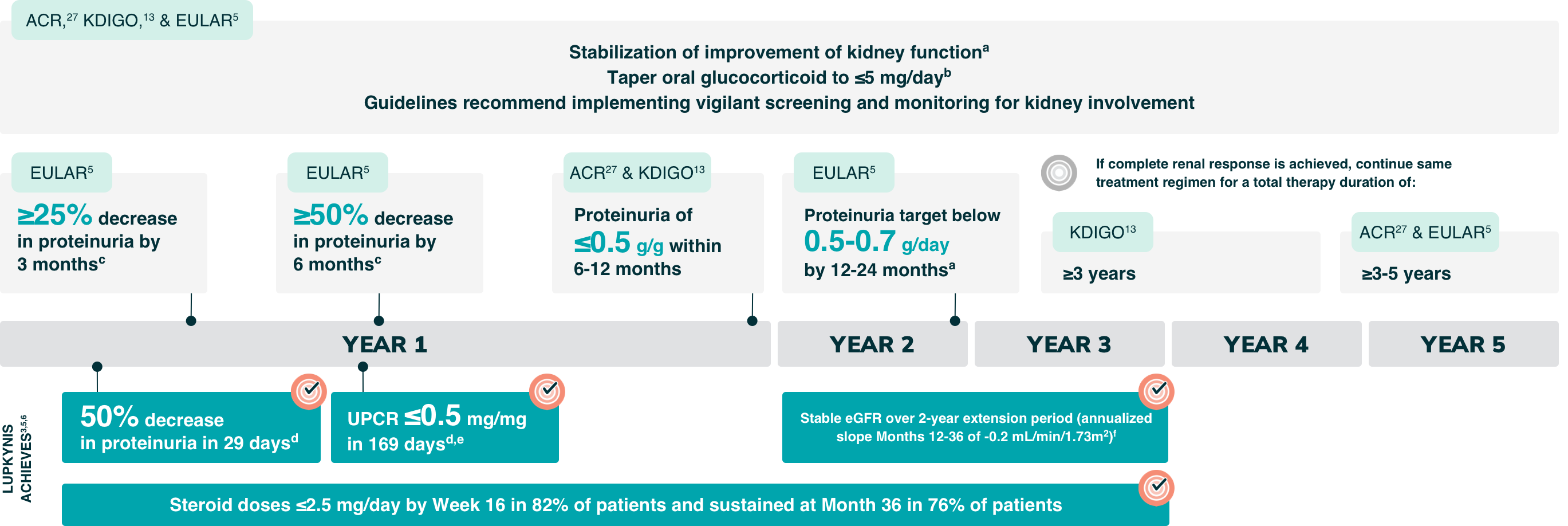
a±20% from baseline for ACR Guideline, ±10-15% from baseline for KDIGO guideline, and ±10% from baseline for EULAR guideline.5,13,27
bBy 6 months for ACR and KDIGO guidelines, and as quickly as possible for EULAR guideline.5,13,27
cAll with eGFR within 10% from baseline.5
dMedian time.
ePatients in the LUPKYNIS arm were more likely than those in the active control arm to achieve 50% UPCR reduction in <1 month (29 days vs. 63 days, respectively) and UPCR ≤0.5 mg/mg in <6 months (169 days vs. 372 days, respectively; HR: 2.0; 95% CI: 1.5-2.7).4,5
fThe annualized corrected eGFR slope Years 2-3 with LUPKYNIS and MMF + low-dose steroids (n=114) was -0.2 mL/min/1.73 m2 (95% CI: -3.0 to 2.7) and with placebo + MMF + low-dose steroids alone (n=100) was -5.4 mL/min/1.73 m2 (95% CI: -8.4 to -2.3).6
Summary of 2024 ACR Guideline for Treatment of LN
A triple immunosuppressive regimen is the preferred first-line therapy for LN



Review this comprehensive video walkthrough of the 2024 ACR LN Guideline
DOWNLOAD THE GUIDELINE (PDF)aDiscuss adjunctive treatment with systemic anticoagulation with nephrology for patients with LN and significant risk factors for thrombosis (e.g., low serum albumin in context of severe proteinuria).1
bFor ≥1 g protein; for <1 g, treat with glucocorticoid and/or immunosuppression.1
cSubstitute MPAA once LD CYC cycle is completed.1
dFor people with extrarenal manifestations, triple therapy containing belimumab is recommended.1
eFor people with proteinuria ≥3 g/g, triple therapy containing a CNI (e.g., LUPKYNIS) is recommended.1
EULAR Guideline: Recommended Management Targets for LN
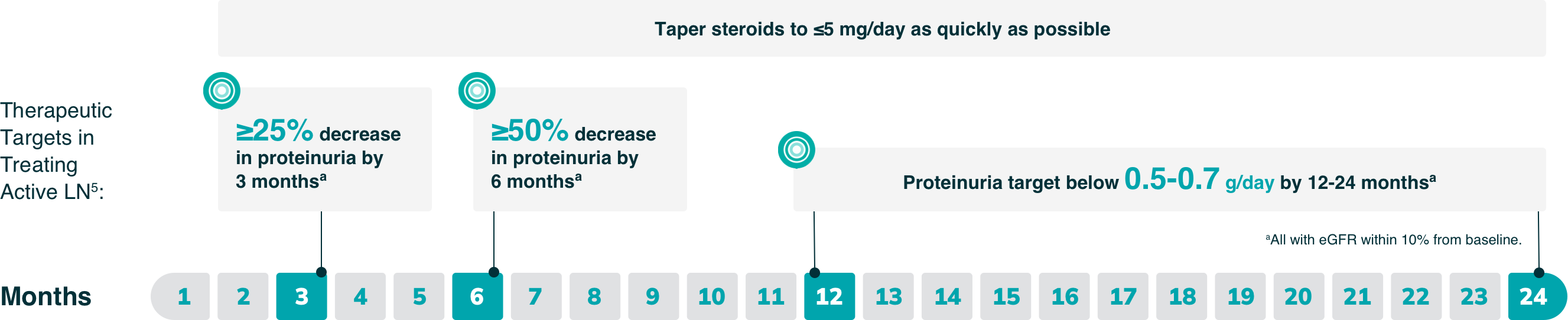
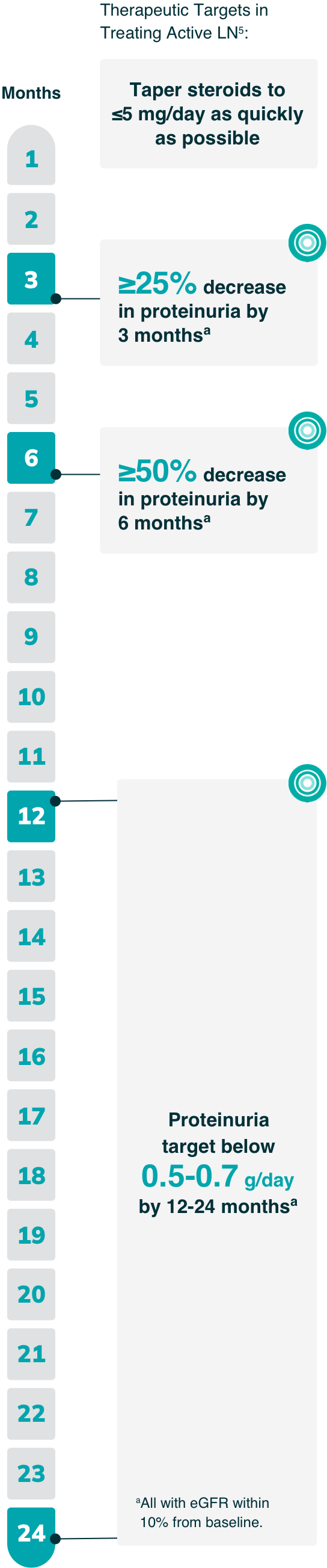
As part of a comprehensive approach to preserving kidney function in patients with LN, the 2023 EULAR guideline outlines validated thresholds for evidence of improvement in proteinuria5
- By 3 and 6 months, a partial response (25% and 50% reductions in proteinuria, respectively) is the recommended goal, while a complete clinical response (<0.5 to 0.7 g/day) is the goal by 12-24 months (all with eGFR within 10% of baseline)
This guideline reflects increasing evidence that even low levels of proteinuria can contribute to chronic, irreversible kidney damage.5
IMPORTANT NOTE: These are only select recommendations, not the complete EULAR recommendations.

Duration of therapy: Continue therapy for 3-5 years following renal response5
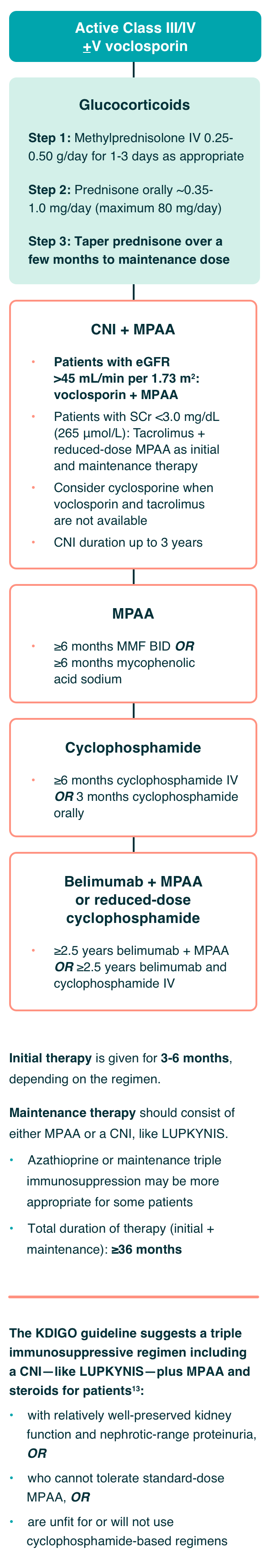
Recommendations for the Management of Patients With Class III or IV LN13:
Severe disease resulting in acute kidney injury which can lead to permanent nephron loss
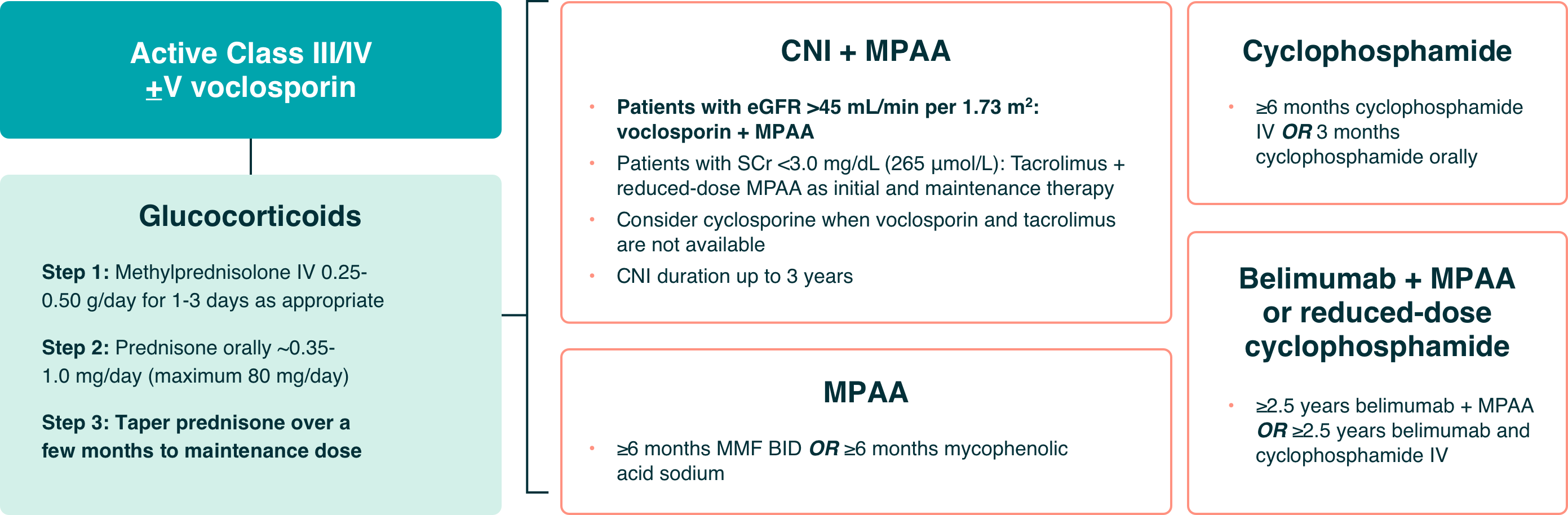
Initial therapy is given for 3-6 months, depending on the regimen.
Maintenance therapy should consist of either MPAA or a CNI, like LUPKYNIS.
- Azathioprine or maintenance triple immunosuppression may be more appropriate for some patients
- Total duration of therapy (initial + maintenance): ≥36 months
The KDIGO guideline suggests a triple immunosuppressive regimen including a CNI—like LUPKYNIS—plus MPAA and steroids for patients13:
- with relatively well-preserved kidney function and nephrotic-range proteinuria, OR
- who cannot tolerate standard-dose MPAA, OR
- are unfit for or will not use cyclophosphamide-based regimens
Based on evidence from AURA-LV and AURORA 1, the authors of the KDIGO 2024 Clinical Practice Guideline for the Management of Lupus Nephritis ascertained that LUPKYNIS increases complete renal remissions with a high level of certainty, citing the effect of LUPKYNIS on complete renal remissions as large and significant.13
LUPKYNIS Exceeds Guideline Goals With Rapid Reduction of Proteinuria and Steroid Doses1-6
The 2024 ACR Guideline, 2024 KDIGO Guideline, and 2023 EULAR Guideline for LN Recommend5,13,27:

a±20% from baseline for ACR Guideline, ±10-15% from baseline for KDIGO guideline, and ±10% from baseline for EULAR guideline.5,13,27
bBy 6 months for ACR and KDIGO guidelines, and as quickly as possible for EULAR guideline.5,13,27
cAll with eGFR within 10% from baseline.5
dMedian time.
ePatients in the LUPKYNIS arm were more likely than those in the active control arm to achieve 50% UPCR reduction in <1 month (29 days vs. 63 days, respectively) and UPCR ≤0.5 mg/mg in <6 months (169 days vs. 372 days, respectively; HR: 2.0; 95% CI: 1.5-2.7).4,5
fThe annualized corrected eGFR slope Years 2-3 with LUPKYNIS and MMF + low-dose steroids (n=114) was -0.2 mL/min/1.73 m2 (95% CI: -3.0 to 2.7) and with placebo + MMF + low-dose steroids alone (n=100) was -5.4 mL/min/1.73 m2 (95% CI: -8.4 to -2.3).6
Summary of 2024 ACR Guideline for Treatment of LN
A triple immunosuppressive regimen is the preferred first-line therapy for LN

Review this comprehensive video walkthrough of the 2024 ACR LN Guideline


aDiscuss adjunctive treatment with systemic anticoagulation with nephrology for patients with LN and significant risk factors for thrombosis (e.g., low serum albumin in context of severe proteinuria).1
bFor ≥1 g protein; for <1 g, treat with glucocorticoid and/or immunosuppression.1
cSubstitute MPAA once LD CYC cycle is completed.1
dFor people with extrarenal manifestations, triple therapy containing belimumab is recommended.1
eFor people with proteinuria ≥3 g/g, triple therapy containing a CNI (e.g., LUPKYNIS) is recommended.1
EULAR Guideline: Recommended Management Targets for LN
As part of a comprehensive approach to preserving kidney function in patients with LN, the 2023 EULAR guideline outlines validated thresholds for evidence of improvement in proteinuria5
- By 3 and 6 months, a partial response (25% and 50% reductions in proteinuria, respectively) is the recommended goal, while a complete clinical response (<0.5 to 0.7 g/day) is the goal by 12-24 months (all with eGFR within 10% of baseline)
This guideline reflects increasing evidence that even low levels of proteinuria can contribute to chronic, irreversible kidney damage.5
IMPORTANT NOTE: These are only select recommendations, not the complete EULAR recommendations.

Duration of therapy: Continue therapy for 3-5 years following renal response5
Recommendations for the Management of Patients With Class III or IV LN13:
Severe disease resulting in acute kidney injury which can lead to permanent nephron loss
Based on evidence from AURA-LV and AURORA 1, the authors of the KDIGO 2024 Clinical Practice Guideline for the Management of Lupus Nephritis ascertained that LUPKYNIS increases complete renal remissions with a high level of certainty, citing the effect of LUPKYNIS on complete renal remissions as large and significant.13
Connect with an Aurinia representative.
Sign up for the latest news, updates, and materials for LUPKYNIS.
GET CONNECTED
ACR=American College of Rheumatology; aPL=antiphospholipid antibody; BEL=belimumab; BID=twice daily; CI=confidence interval; CKD=chronic kidney disease; CNI=calcineurin inhibitor; eGFR=estimated glomerular filtration rate; ESKD=end-stage kidney disease; EULAR=European Alliance of Associations for Rheumatology; GC=glucocorticoid; hpf=high-power field; HR=hazard ratio; ISN/RPS=International Society of Nephrology/Renal Pathology Society; IV=intravenous; KDIGO=Kidney Disease: Improving Global Outcomes; LD CYC=low-dose cyclophosphamide; LN=lupus nephritis; MMF=mycophenolate mofetil; MPAA=mycophenolic acid analogs; OR=odds ratio; RAAS-I=renin-angiotensin-aldosterone system inhibitor; RBC=red blood cell; SCr=serum creatinine; SLE=systemic lupus erythematosus; UPCR=urine protein-to-creatinine ratio; WBC=white blood cell.
References: 1. Yurkovich M, Vostretsova K, Chen W, Aviña-Zubieta JA. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res (Hoboken). 2014;66(4):608-616. 2. Hanly JG, O’Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatol. 2016;55(2):252-262. 3. Bertsias GK, Tektonidou M, Amoura Z, et al. Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012;71(11):1771-1782. 4. Hermansen ML, Jesper Lindhardsen J, Torp-Pedersen C, Faurschou M, and Jacobsen S. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology (Oxford). 2017;56(5):709-715. 5. Fanouriakis A, Kostopoulou M, Andersen J, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. 2024;83(1):15-29. 6. Anders HJ, Saxena R, Zhao M, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. 2020;6(1):7. 7. Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley K, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia Lupus Registry. Arthritis Rheumatol. 2014;66(2):357-368. 8. Somers EC, Marder W, Cagnoli P, et al. Population-based incidence and prevalence of systemic lupus erythematosus: The Michigan Lupus Epidemiology and Surveillance Program. Arthritis Rheumatol. 2014;66(2):369-378. 9. Izmirly PM, Wan I, Sahl S, et al. The incidence and prevalence of systemic lupus erythematosus in New York County (Manhattan), New York. Arthritis Rheumatol. 2017;69(10):2006–2017. 10. Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention national lupus registries. Arthritis Rheumatol. 2021;73(6):991-996. 11. Dall’Era M, Cisternas MG, Snipes K, Herrinton LJ, Gordon C, Helmick CG. The incidence and prevalence of systemic lupus erythematosus in San Francisco County, California: The California Lupus Surveillance Project. Arthritis Rheumatol. 2017;69(10):1996-2005. 12. Aggarwal I, Li J, Trupin L, et al. Quality of care for the screening, diagnosis, and management of lupus nephritis across multiple healthcare settings. Arthritis Care Res (Hoboken). 2020;72(7):888-896. 13. KDIGO Lupus Nephritis Work Group. KDIGO 2024 clinical practice guideline for the management of lupus nephritis. Kidney Int. 2024;105(1S):S1-S69. 14. De Rosa M, Rocha AS, De Rosa G, Dubinsky D, Almaani SJ, Rovin BH. Low-grade proteinuria does not exclude significant kidney injury in lupus nephritis. Kidney Int Rep. 2020;5(7):1066-1068. 15. Albuquerque BC, Salles VB, de Paulo Tajra RD, Rodrigues CEM. Outcome and prognosis of patients with lupus nephritis submitted to renal transplantation. Sci Rep. 2019;9(1):11611. 16. Cravedi P, Remuzzi G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br J Clin Pharmacol. 2013;76(4):516-523. 17. Koo HS, Kim S, Chin HJ. Remission of proteinuria indicates good prognosis in patients with diffuse proliferative lupus nephritis. Lupus. 2016;25(1):3-11. 18. Ugolini-Lopes MR, Seguro LPC, Castro MXF, et al. Early proteinuria response: a valid real-life situation predictor of long-term lupus renal outcome in an ethnically diverse group with severe biopsy-proven nephritis? Lupus Sci Med. 2017;4(1):e000213. 19. Tamirou F, Lauwerys BR, Dall’Era M, et al. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN Nephritis Trial. Lupus Sci Med. 2015;2(1):e000123. 20. Moroni G, Gatto M, Tamborini F, et al. Lack of EULAR/ERA-EDTA response at 1 year predicts poor long-term renal outcome in patients with lupus nephritis. Ann Rheum Dis. 2020;79(8):1077-1083. 21. Ding JYC, Ibañez D, Gladman DD, Urowitz MB. Isolated hematuria and sterile pyuria may indicate systemic lupus erythematosus activity. J Rheumatol. 2015;42(3):437-440. 22. Christopher-Stine L, Siedner M, Lin J, et al. Renal biopsy in lupus patients with low levels of proteinuria. J Rheumatol. 2007,34(2):332-335. 23. Maria NI, Davidson A. Protecting the kidney in systemic lupus erythematosus: from diagnosis to therapy. Nat Rev Rheumatol. 2020;16(5):255-267. 24 Chen YE, Korbet SM, Katz RS, Schwartz MM, Lewis EJ, for the Collaborative Study Group. Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol. 2008;3(1):46-53. 25. Appel GB, Contreras G, Dooley MA, et al, and the Aspreva Lupus Management Study Group. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103-1112. 26. Saxena A, Ginzler EM, Gibson K, et al. Safety and efficacy of long-term voclosporin treatment for lupus nephritis in the phase 3 AURORA 2 clinical trial. Arthritis Rheumatol. 2024;76(1):59-67. 27. 2024 American College of Rheumatology (ACR) guideline for the screening, treatment, and management of lupus nephritis: guideline summary. November 18, 2024. 28. LUPKYNIS®. Prescribing information. Aurinia Pharma U.S., Inc.; 2025.